Lewis Structure Of Sf4 slidesharetrick
Steps of drawing SF4 lewis structure Step 1: Find the total valence electrons in SF4 molecule. In order to find the total valence electrons in SF4 molecule, first of all you should know the valence electrons present in sulfur atom as well as fluorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily find.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
I quickly take you through how to draw the Lewis Structure of SF4, Sulfur TetraFluoride. I also go over formal charge, hybridization, shape and bond angle.**.
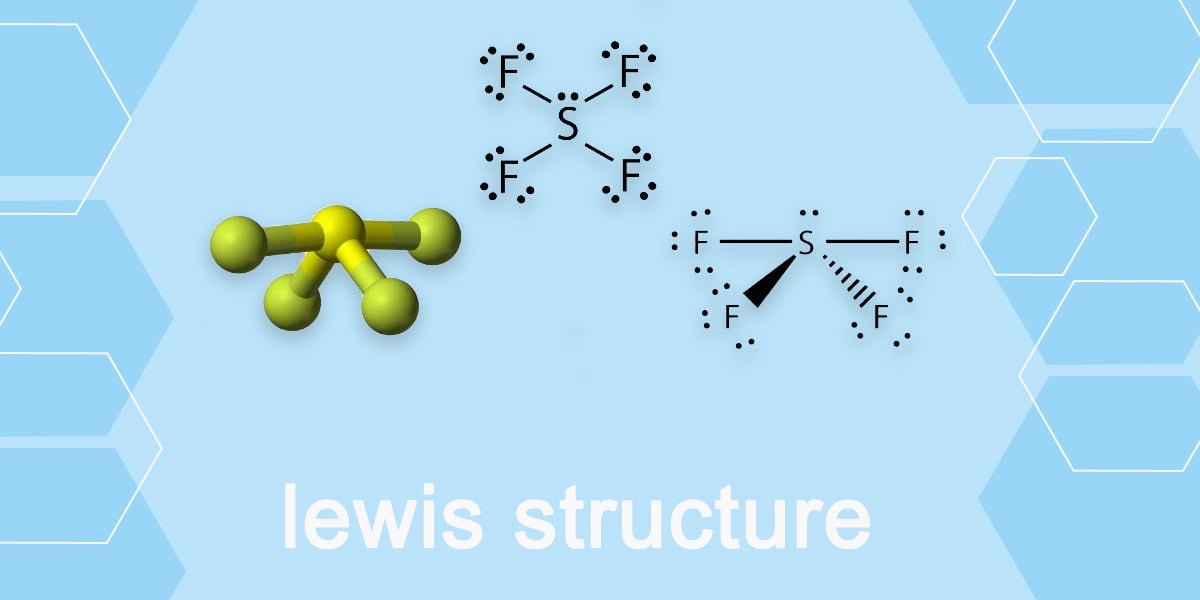
How to draw Sf4 Lewis Structure? Beginners Guide
The Lewis structure of SF4 indicates five regions of electron density around the sulfur atom: one lone pair and four bonding pairs: We expect these five regions to adopt a trigonal bipyramidal electron-pair geometry. To minimize lone pair repulsions, the lone pair occupies one of the equatorial positions..

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two. So, it will lie at the center of the molecule. Dash lines represent the four S-F single covalent bonds. Dots represent the lone pairs. VSEPR theory is used to predict the shape of the SF 4 molecule. According to this.
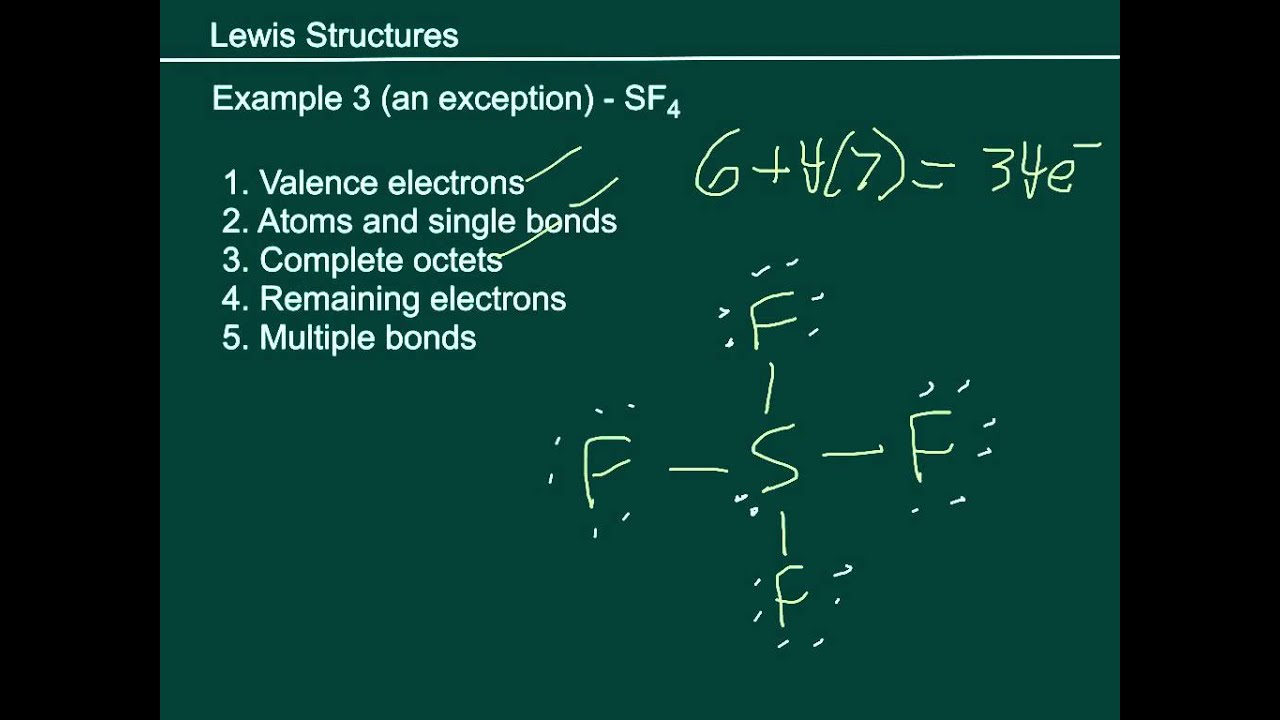
Basic Lewis Structures SF4 YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SF4. In order to draw the lewis structure of SF4, first of all you have to find the total number of valence electrons present in the SF4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

SF4 Molecular Geometry Science Education and Tutorials
In SF4 lewis structure, each fluorine atom has joint with center sulfur atom. Also, there is a lone pair on sulfur atom. Sulfur has a valence of 6. Therefore sulfur becomes the center atom. In this tutorial, we will learn how to draw the lewis structure of SF4 step by step.
[Solved] Draw the Lewis structure of SF 4 showing all lone pairs. Identify... Course Hero
To draw the Lewis structure of SF4, we follow a step-by-step process. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the atoms to form bonds and lone pairs. 1. Determine the Total Number of Valence Electrons. To begin, count the total number of valence electrons in the SF4 molecule.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure, there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs.
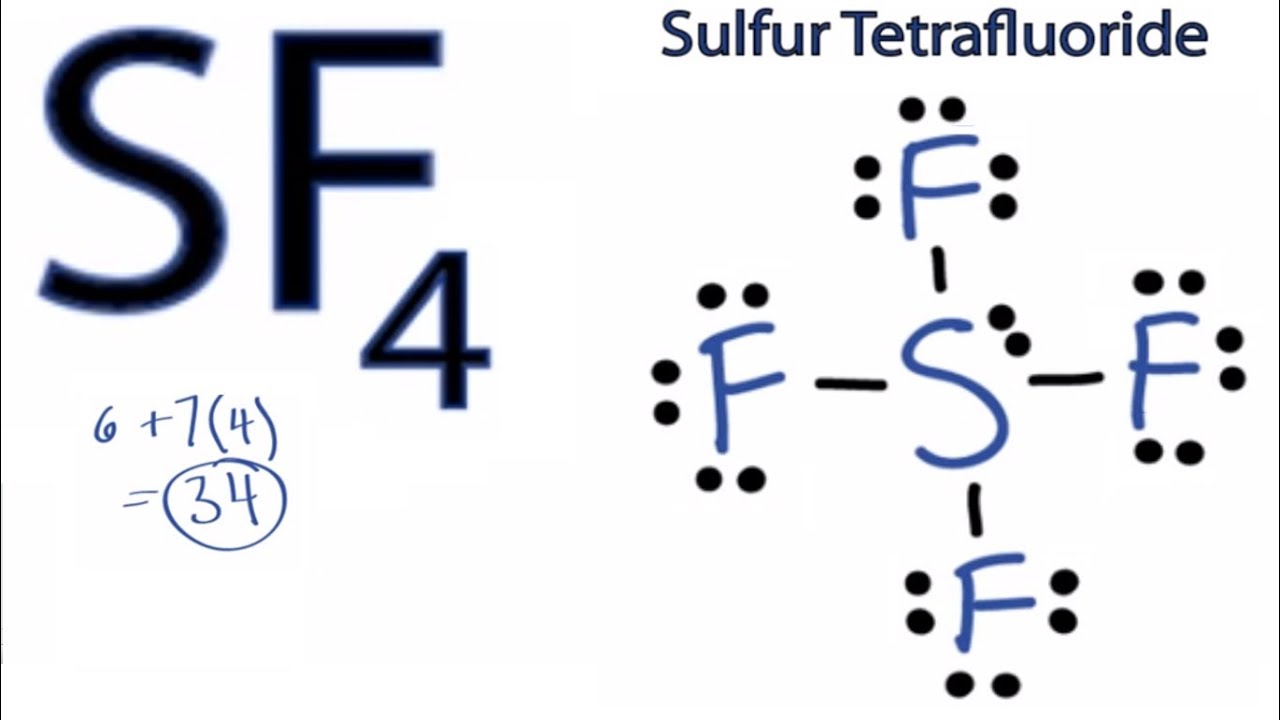
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
The Lewis structure helps one to understand the sharing of electrons between the central and neighboring atoms within a compound. Here the central atom is Sulfur and the neighboring atoms are Fluorine. This is the general idea of how and why Lewis structures are made. SF4 Lewis Structure. Let us look at how SF4's Lewis structure can be formed.

Sf4 Polar or Nonpolar
For the SF4 Lewis structure use the periodic table to find the total number of valence electrons for the SF4 molecule. Once we know how many valence electrons there are in SF4 we can distribute them around the central atom with the goal of filling the outer shells of each atom. Note that Sulfur is the least electronegative element in the SF4.
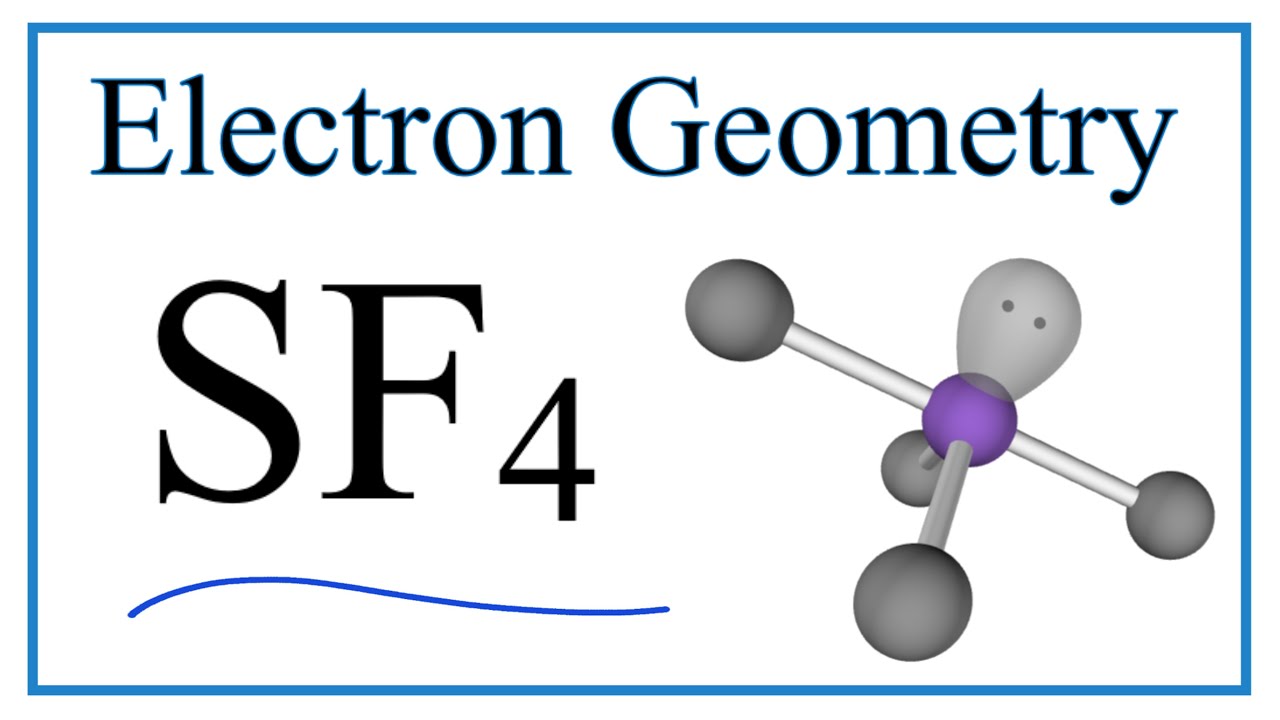
SF4 Electron Geometry (Sulfur tetrafluoride) YouTube
A step-by-step explanation of how to draw the SF4 Lewis Structure (Sulfur Tetrafluoride).For the SF4 Lewis structure use the periodic table to find the total.

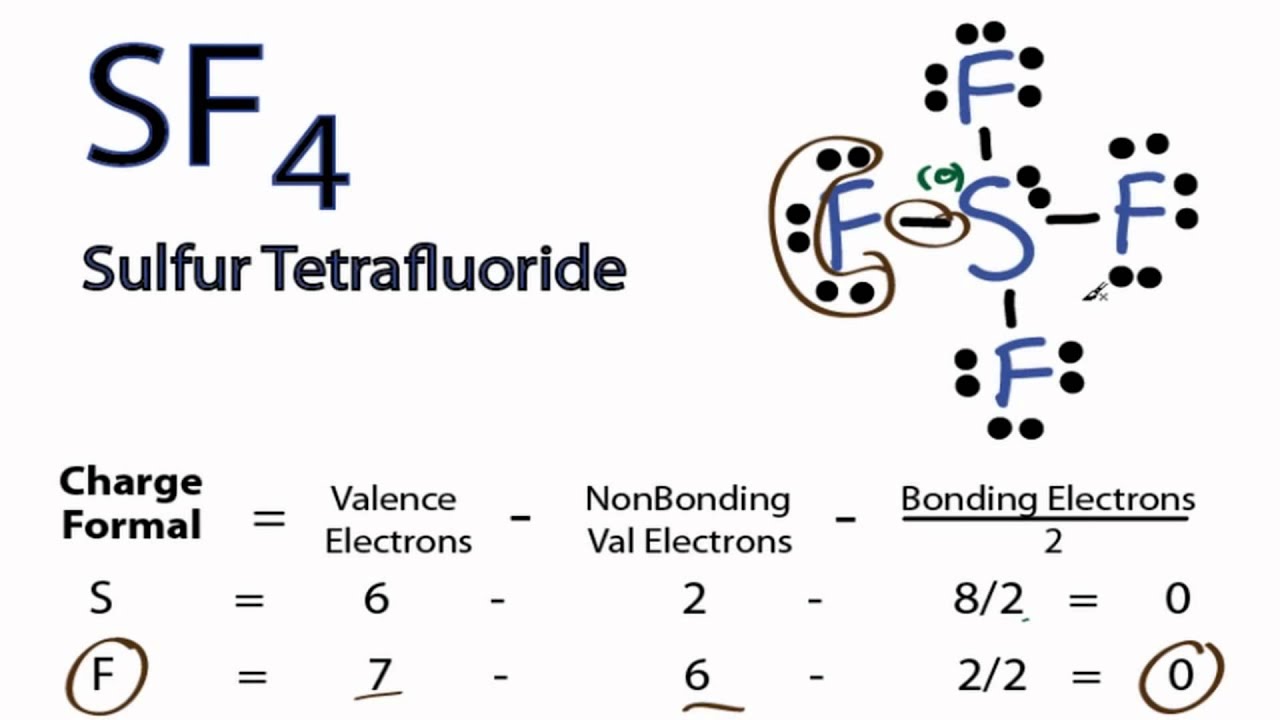
SF4 Lewis Structure ,Valence Electrons,Formal Charge,Octet Rule
SF4 Lewis Structure A. Definition and concept. The SF4 Lewis structure is a diagram that represents the arrangement of atoms and electrons in the molecule. The concept of valence electrons, which participate in chemical bonding as the outermost electrons, serves as the basis for it. The structure shows the central sulfur atom bonded to four.
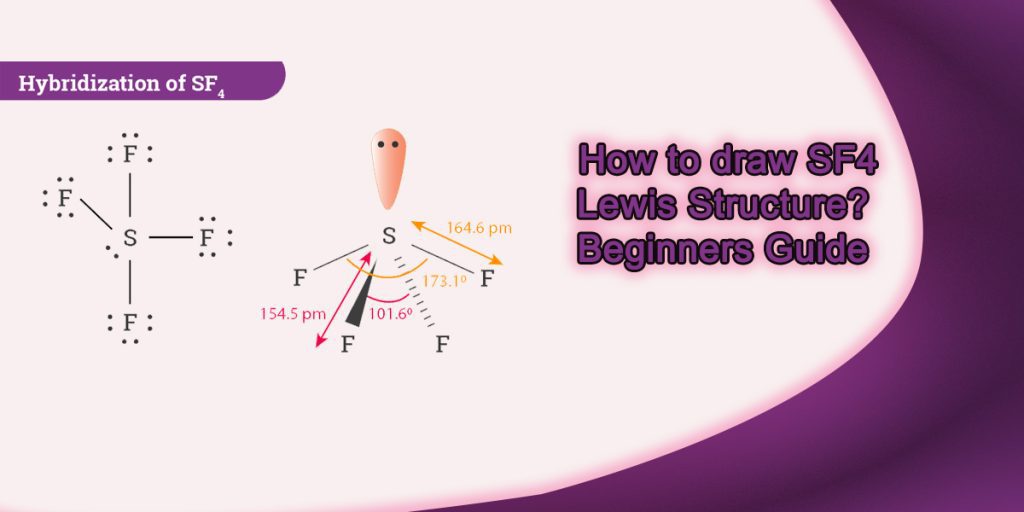
How to draw Sf4 Lewis Structure? Beginners Guide
The SF4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF4 molecule. The geometry of the SF4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the SF4 geometrical shape in which the electrons.

How to draw SF4 Lewis Structure? Science Education and Tutorials
Sulfur tetrafluoride is the chemical compound with the formula S F 4.It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

SF4 Lewis Structure ,Valence Electrons,Formal Charge,Octet Rule
A step-by-step explanation of how to draw the SF4 Lewis Dot Structure (Sulfur tetrafluoride).For the SF4 structure use the periodic table to find the total n.
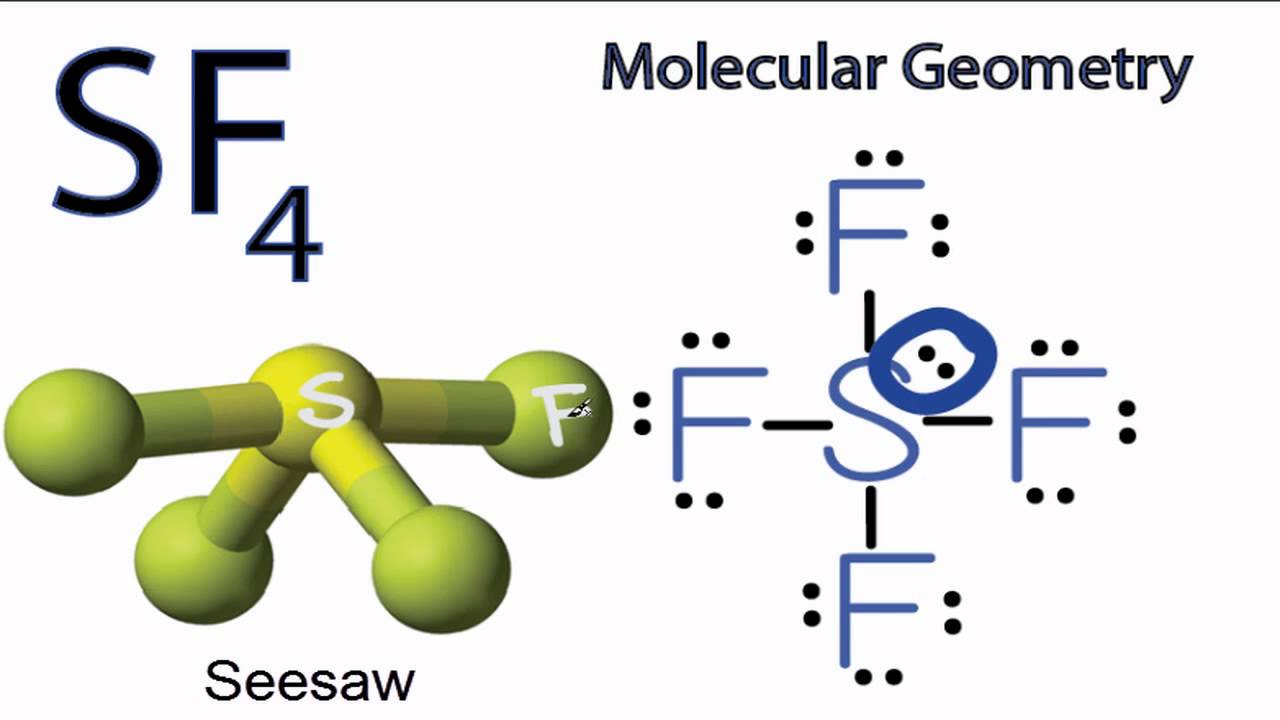
SF4 Molecular Geometry / Shape YouTube
Sulfur tetrafluoride (SF4) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge. SF 4 is the chemical formula for sulfur tetrafluoride, a colorless gas with a distinct rotten-egg-like odor. Its molar mass is 108.4 g/mol thus it is heavier than air. Sulfur tetrafluoride is a highly toxic gas that can.